-
Ordered and Oriented Supramolecular n/p-Heterojunction Surface Architectures: Completion of the Primary Color Collection
R.S.K. Kishore, O. Kel, N. Banerji, D. Emery, G. Bollot, J. Mareda, A. Gomez-Casado, P. Jonkheijm, J. Huskens, P. Maroni, M. Borkovec, E. Vauthey, N. Sakai and S. Matile
Journal of the American Chemical Society, 131 (31) (2009), p11106-11116


DOI:10.1021/ja9030648 | unige:6173 | Abstract | Article HTML | Article PDF
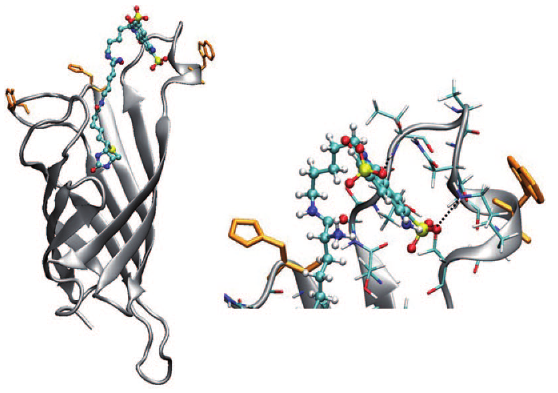
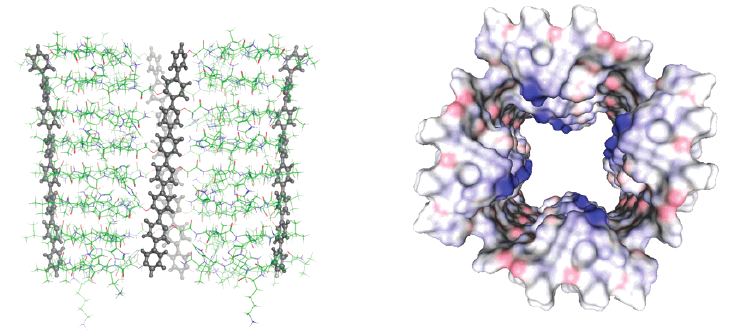
In this study, we describe synthesis, characterization, and zipper assembly of yellow p-oligophenyl naphthalenediimide (POP-NDI) donorâacceptor hybrids. Moreover, we disclose, for the first time, results from the functional comparison of zipper and layer-by-layer (LBL) assembly as well as quartz crystal microbalance (QCM), atomic force microscopy (AFM), and molecular modeling data on zipper assembly. Compared to the previously reported blue and red NDIs, yellow NDIs are more Ï-acidic, easier to reduce, and harder to oxidize. The optoelectronic matching achieved in yellow POP-NDIs is reflected in quantitative and long-lived photoinduced charge separation, comparable to their red and much better than their blue counterparts. The direct comparison of zipper and LBL assemblies reveals that yellow zippers generate more photocurrent than blue zippers as well as LBL photosystems. Continuing linear growth found in QCM measurements demonstrates that photocurrent saturation at the critical assembly thickness occurs because more charges start to recombine before reaching the electrodes and not because of discontinued assembly. The found characteristics, such as significant critical thickness, strong photocurrents, large fill factors, and, according to AFM images, smooth surfaces, are important for optoelectronic performance and support the existence of highly ordered architectures.